2019-nCoV lgG/lgM Rapid One Step Rapid Test Device COVID-19 Rapid Test Kits
- Model
- HCS 003
Item specifics
- Name
- COVID-19 lgG/lgM Rapid Test Kits
- Cat.
- COV-102
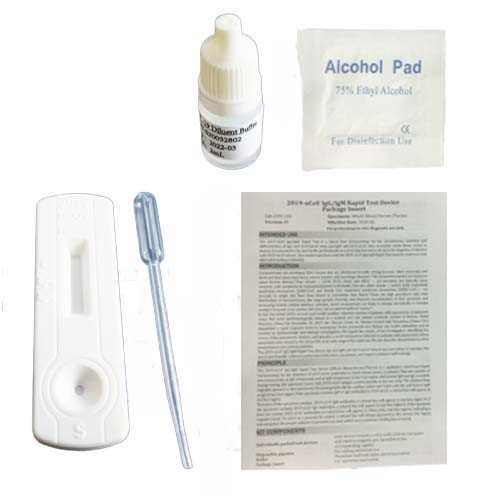
- Speciments
- Whole Blood / Serum / Plasma
- QTY
- 25 PCS Devices
- Aluminum Foil Bag
- 120*65mm
- Box
- 190*125*70mm
- Package Case
- 395*515*375mm
- Box Weight
- 220g
- Case Weight
- 10kg
Review
Description
INFORMATIONS
- Name
- COVID-19 lgG/lgM Rapid Test Kits
- Cat.
- COV-102
- Speciments
- Whole Blood / Serum / Plasma
- QTY
- 25 PCS Devices
- Aluminum Foil Bag
- 120*65mm
- Box
- 190*125*70mm
- Box Weight
- 220g
- Package Case
- 395*515*375mm
WARNINGS AND PRECAUTIONS
- For professional in vitro diagnostic use only. Do not use after expiration date.
- Do not eat,drink or smoke in the area where the specimens or kits are handled.
- Handle all specimens as if they contain infectious agents.
- Observe established precautions microbiological hazards throughout testing and follow the standard procedures for proper of specimens.
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
- Humidity and temperature can adversely affect results.
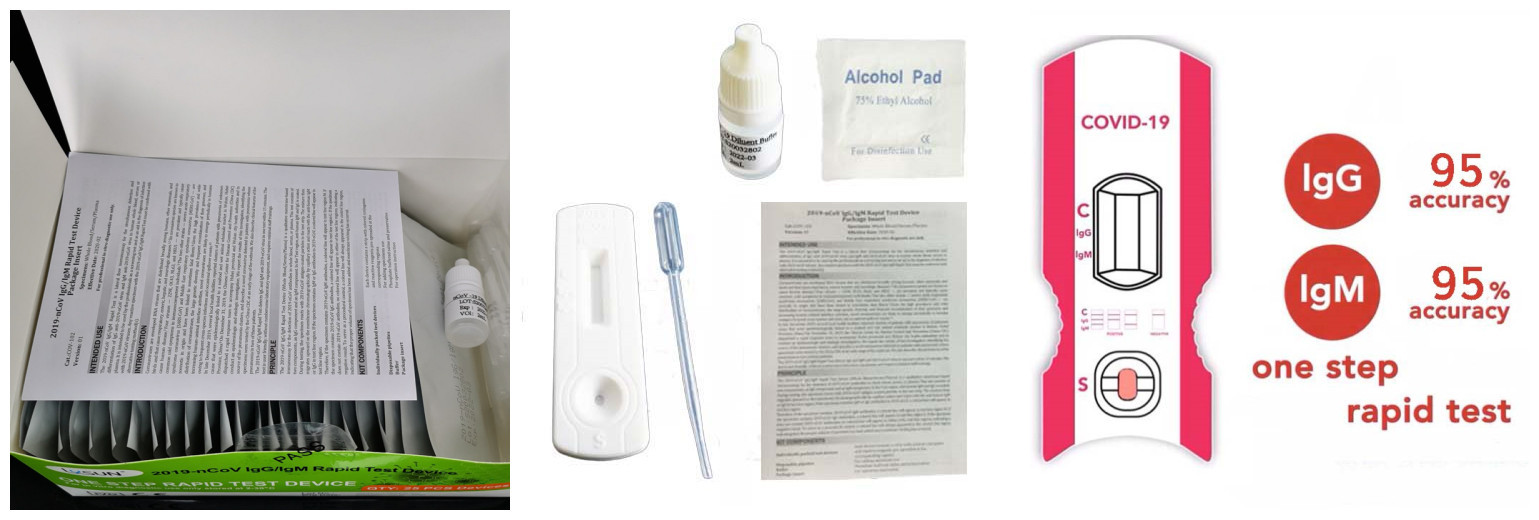
STEPS FOR USAGE
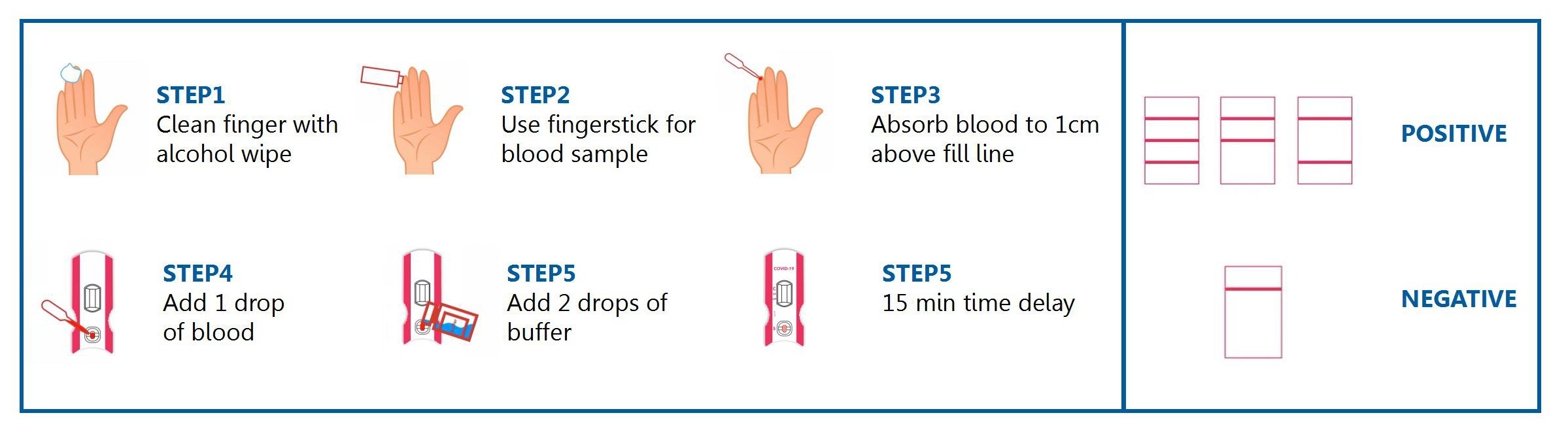
The "C" line will definitely appear. If it does not appear,the operation is incorrect.
The lgM line shows that the person being tested has been infected with the COVID-19 virus for 3-7 days
The lgG line shows that the person being tested has been infected with the COVID-19 virus for more than 7 days
PRODUCT

CERTIFICATIONS(CE,EN ISO13485)

This brand was registered in the Dutch Drug Administration (CIBG)